They may sound similar, but losing weight and eliminating fat are actually 2 different things.
Losing weight doesn’t always lead to a more sculpted appearance, but eliminating treated fat cells can change the shape and contours of your body.4

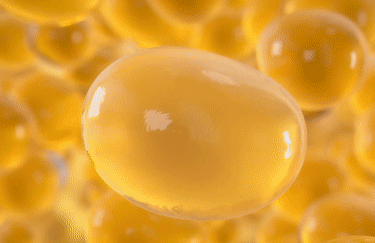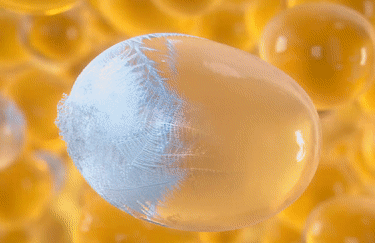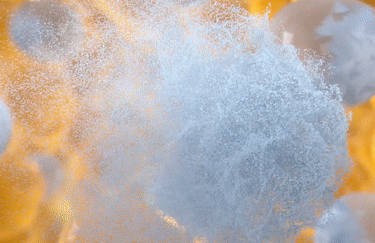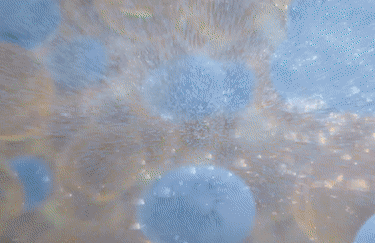
All about fat cells
The number of fat cells varies from person to person, but it doesn't change much once you're an adult.16 You have a certain number of fat cells, and they expand or shrink according to your diet and exercise routine. Some people may have stubborn pockets of fat that are more difficult to reduce despite diet and exercise.



CoolSculpting® vs stubborn fat areas
CoolSculpting® Elite targets, freezes, and eliminates treated fat cells using fat-freezing technology known as cryolipolysis. This non-invasive process naturally eliminates up to 27% of treated fat cells over a 1-3 month periods.6,10



Pinpoint your
trouble spots
With CoolSculpting® Elite, you can pinpoint exact trouble spots where you’d like to reduce fat. You will work directly with a provider to customize a treatment plan that’s unique to you and your body contouring goals.
FIND A CLINICReady to discover if CoolSculpting® Elite is right for you?
 Find
a Clinic
Find
a Clinic
Intended Use:
The CoolSculpting® Elite System is a non-invasive cooling and heating device that applies controlled cooling or heating to a treatment site on the patient's skin. Uses of the system in cooling mode include:
- Fat layer reduction through cold assisted lipolysis.
The optional massage function can also be used for temporary: - Reduction in the appearance of cellulite.
Important safety information:
During the procedure patients may experience sensations of pulling, tugging, mild pinching, intense cold, tingling, stinging, aching and cramping at the treatment site.13 These sensations subside as the area becomes numb.13
Following the procedure, typical side effects include redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, skin sensitivity and numbness. Numbness can persist for up to several weeks. A sensation of fullness in the back of the throat may occur after submental treatment. The following rare and very rare side effects have the following incidence rates (approximate occurrences per number of treatments): paradoxical hyperplasia (1/3000 [0.033%]); late-onset pain (1/6000 [0.017%]); severe pain (1/6000 [0.017%]); hyperpigmentation (1/11000 [0.009%]); freeze burn (1/15000 [0.006%]); treatment area demarcation (1/20000 [0.005%]); vasovagal symptoms (1/30000 [0.003%]); subcutaneous induration (1/30000 [0.003%]); cold panniculitis (1/60000 [0.002%]) and hernia (1/185000 [0.001%]).13,15 The CoolSculpting® procedure is not for everyone. Patients should not have the CoolSculpting® procedure if they suffer from cryoglobulinaemia, cold agglutinin disease or paroxysmal cold haemoglobinuria.13 The CoolSculpting® procedure is not a treatment for obesity.14 CoolSculpting® is not a weight loss procedure and should not replace a healthy diet and active lifestyle.
Please see CoolSculpting® and CoolSculpting Elite® Important Safety Information for additional information.
Effect of treatment varies with individuals
References:
- Medical Insight Inc. The Global Aesthetic Market Study: Version XVII. 2019
- All time International Cycles Summary_040621. Data sourced from Connect Cube database [provided by International Business Excellence]. May 2021.
- Manstein D et al. Lasers Surg Med 2008; 40: 595-604
- Zelickson B, et al. Derm Surg 2009; 35:1462-70
- Allergan Inc. Unpublished Data. CoolSculpting® Publications. INT-CSC-2050471. December 2020.
- Sasaki GH, et al. Aesthet Surg J 2014; 34:420–31
- Allergan. Unpublished data. INT-CSC-2050028. CoolSculpting® clinical “Fit and Function” study to test the V003 wells. February 2020.
- Allergan. Unpublished data. INT-CSC-2050288. Applicator cooling and distribution dye test. August 2020.EN-A.
- CoolSculpting® System (CoolSculpting® Elite) user manual. CS-UM-CM3-07-EN-E. Feb 2023.
- Manstein D et al. Lasers Surg Med 2008; 40: 595-604
- Avram MM and Harry RS. Lasers Surg Med 2009; 41:703-8
- Krueger N, et al. Clin Cosmet Investig Derm 2014; 7:201-25
- CoolSculpting® system user manual. BRZ-101-TUM-SGP-B COM1. Sep 2021
- FDA 501(K) Summary Document, Available at : http://www.accessdata.fda.gov/ [Accessed February 2022].
- Allergan, Unpublished date, INT-CSC-2050028, CoolSculpting, clinical fit and function study testing of V003 cups, February 2020.
- Spalding KL,et al. Nature 2008; 453:783-7.
Disclaimer: CoolSculpting is not a weight loss treatment.
SG-CSC-230058 Date of Approval: October 2023
 About Fat Loss.jpeg)


